SELUTION SLR PTA Drug Eluting Balloon
The SELUTION SLR™ Drug Eluting Balloon (DEB) incorporates the trusted drug, Sirolimus, into innovative balloon technology providing a SOLUTION for peripheral vascular interventions.

Product Description
The New Paradigm for Peripheral Interventions
Breakthrough Technology1 to deliver sustained limus release

CELL ADHERENT TECHNOLOGY
CELL ADHERENT TECHNOLOGY (CAT)™ is a phospholipid blend containing and protecting MicroReservoirs , each incorporated with a 1 μg/mm² Sirolimus dose.
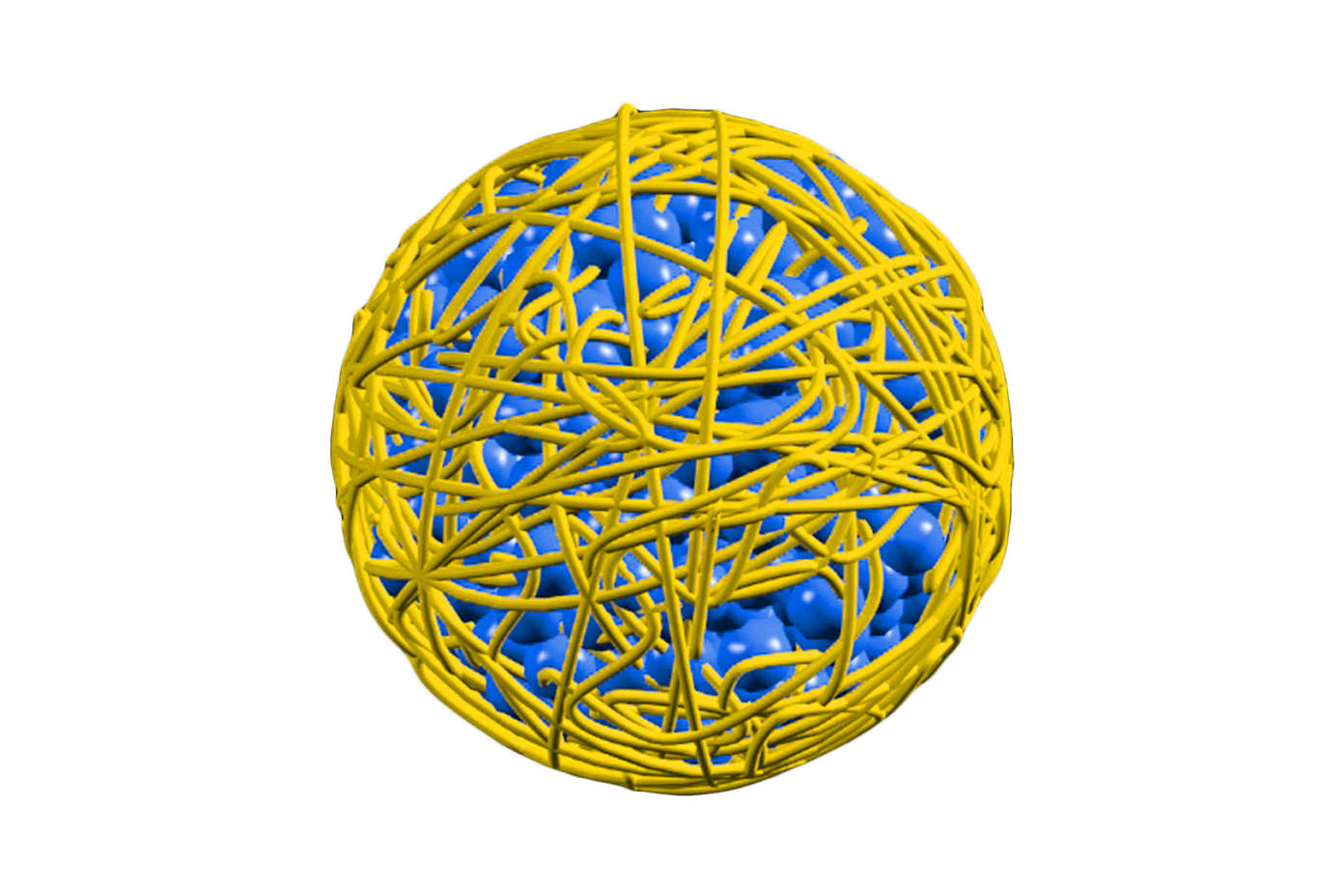
MICRORESERVOIRS
With a single intervention, millions of MicroReservoirs deliver a sustained and consistent drug release at a therapeutic level.

SIZE MATRIX
SELUTION SLR DEB offers a broad portfolio to extend treatment options for peripheral artery disease
1. SELUTION SLR DEB was the first Sirolimus Drug-Eluting Balloon, for BTK, to be awarded Breakthrough Device designation by the US Food and Drug Administration
Advanced Coating Technology

Better Uniformity and Smaller Particle Size2
Durable coating resulting in limited drug loss | CAT contains and protects microreservoirs. The coating is transferred to the vessel wall |
Small homogenous particles | MicroReservoirs (~4 mm) are as small or smaller than red blood cells |
Sirolimus proven safety profile | No local toxic effect on distal tissues / organs |
2. MedAlliance 2024 Data on file.
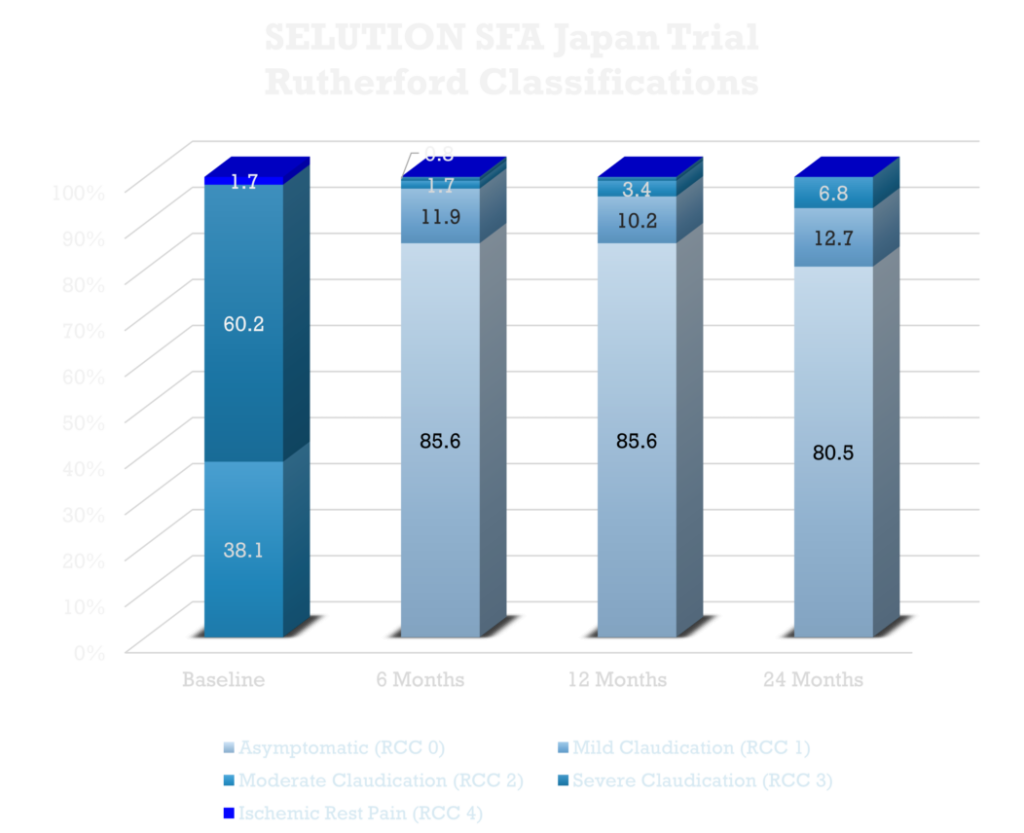
SELUTION SFA Japan Trial3, 4
A rigorous multi-center trial to assess Sirolimus Drug-Eluting Balloon’s efficacy and safety in a Japanese population with symptoms of claudication and rest pain due to femoral-popliteal Peripheral Artery Disease (PAD) with results showing:
- Sustained clinical and hemodynamic improvement through 2 years with a Primary Patency of 83% and freedom from CD TLR of 95.4% (Kaplan–Meier estimate).
- 98.3% of patients had a ≥ 1 category improvement in Rutherford Classification at 2 years, as compared to baseline.
- Results favorably matched best-in-class outcomes from similar studies conducted in and outside Japan.
3. 24-month results from SELUTION SFA Japan Trial, Oral Presentation, JET 2024.
4. Osamu Iida et al, A Novel Sirolimus-Coated Balloon for the Treatment of Femoropopliteal Lesions: The SELUTION SFA Japan Trial, J Am Coll Cardiol Intv. Jun 05, 2024. Epublished DOI: 10.1016/j.jcin.2024.03.029
ADVANCING DRUG-ELUTING BALLOON EDUCATION
Advanced Workshop CLI and Revascularization Management, November 2023, Abano Terme, Italy

How to maximize DEB benefits: a case based practical guide in lower limbs,CIRSE congress, September 2023, Denmark

How I treat CLTI patients with novel Sirolimus DEB: DEB session, PAIRS 2024 conference, February 2024, UAE

Managing CLI patients: From vessel preparation techniques to treatment with sirolimus DEBs, DEB session, LINC congress, May 2024, Germany
Empowering healthcare practitioners through professional education initiatives, workshops and webinars



