MYNX CONTROL Vascular Closure Device
The outstanding design and predictable deployment of MYNX CONTROL™ Vascular Closure Device delivers outstanding performance and control, for reliable secure arterial closures.*
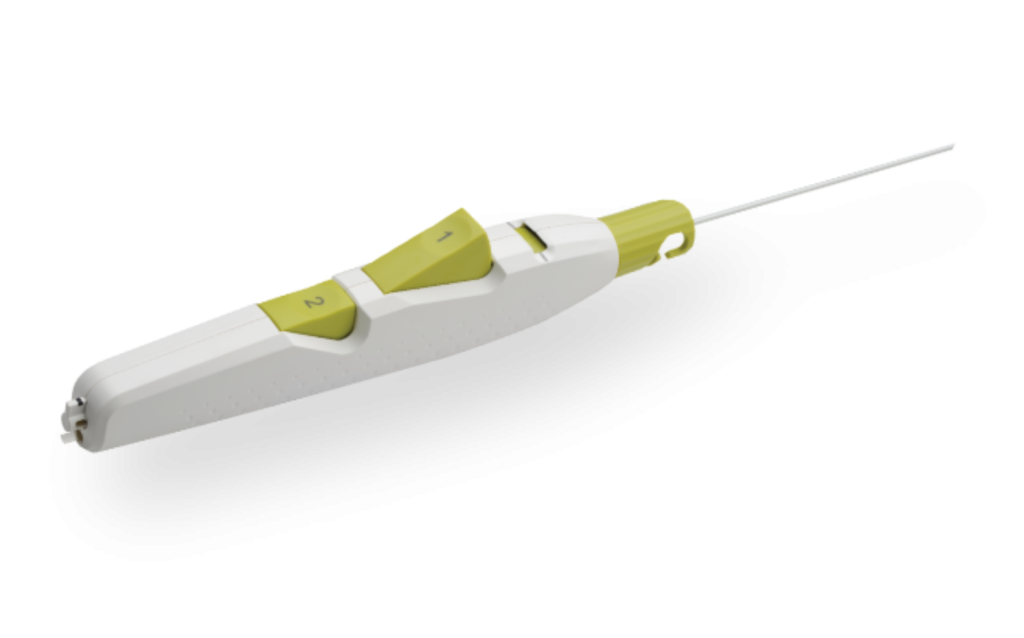
Product Description
MYNX CONTROL Vascular Closure Device integrates active extravascular sealing and resorbability properties with a next-generation delivery system to maximize predictability, safety, and ease of use in sealing 5-7F femoral arterial access sites.
- A next-generation deployment system designed for predictability and ease of use with a 2-button streamline procedural steps
- A sheath catch compatible with the procedural sheath
- A tension indicator providing visual confirmation of device position for proper sealant deployment
- Available in 5F, 6F, and 7F sizes
MYNX™ VCD has been clinically proven to reduce surgical complications, expedite recovery, shorten hospital stays, and increase patient comfort (1-5).
1Pruski MJ Jr et al. MynxGrip for closure of antegrade puncture after peripheral interventions with same-day discharge. Vasc Endovasc Surg. 2017 Feb;51(2):67-71.
2Baker NC et al. Active versus passive anchoring vascular closure devices following percutaneous coronary intervention: a safety and efficacy comparative analysis. J Interv Cardiol. 2016 Feb; 29(1): 108-112.
3Hutchings D et al. Success, safety, and efficacy of the Mynx femoral closure device in a real-world cohort: single-center experience. J Invasive Cardiol. 2016 Mar;28(3):104-108.
4 Noor S et al. Successful reduction of surgeries secondary to arterial access site complications: a retrospective review at a single center with an extravascular closure device. Vasc Endovascular Surg. 2010 Jul;44(5):345-349.
5 Fargen KM, Hoh BL, Mocco J. A prospective randomized single-blind trial of patient comfort following vessel closure: extravascular synthetic sealant closure provides less pain than a self-tightening suture vascular compression device. J NeuroInterv Surg. 2011 Sep; 3(3): 219-223.
The MYNX CONTROL™ VCD includes:
- (1) MYNX CONTROL™ VCD including balloon catheter and integrated polyethylene glycol sealant
- (1) 10 ml locking syringe
| Product | Size | Order Number |
MYNX CONTROL™ VCD | 5F | MX5060E |
| MYNX CONTROL™ VCD | 6F / 7F | MX6760E |


